There are no proven treatments or vaccines for the novel coronavirus which has sickened more than two million people world-wide and killed nearly 150,000 people, but remdesivir is considered a front-runner in the race to develop a treatment for COVID-19 infections that works. Though the findings reported by Stat are promising, they are not based on full clinical trial data from the company.
According to Stat, 125 people with COVID-19 receiving care at the University of Chicago are participating in two Phase 3 clinical trials conducted by Gilead; 113 of them have severe forms of the disease. Stat said it obtained a recorded video discussion about the trial among University of Chicago faculty members in which a physician said when some people start taking the drug, fevers come down and some come off ventilators.
“The best news is that most of our patients have already been discharged, which is great. We’ve only had two patients perish,” University of Chicago infectious disease specialist Kathleen Mullane said, according to STAT News, which obtained a video of her remarks.
One trial is evaluating remdesivir in 2,400 people with severe forms of the disease, the other is testing the drug in 1,600 patients who are moderately ill. Both trials are being conducted at multiple sites around the world. According to ClinicalTrials.gov, both trials began in March and are expected to conclude in May.
In a statement to Stat News on Thursday, Gilead said: “What we can say at this stage is that we look forward to data from ongoing studies becoming available.”
It has been a dramatic week for Gilead’s stock, starting with the release last Friday of a small clinical data set showcasing that 68% of 53 hospitalized patients who had received remdesivir on a compassionate-use basis showed clinical improvement. At that time, a J.P. Morgan analyst called the data “a promising first look” but warned the results “need to be kept in context.”
On Wednesday, shares tumbled after a clinical-trial listing was updated to say Chinese authorities had stopped recruiting for a remdesivir clinical trial in severely ill COVID-19 patients there. Shares rose again on a Bloomberg News report that Gilead is considering taking a stake in Arcus Biosciences Inc. (RCUS).
So, is this it? Is the end of the coronavirus crisis simply a matter of Gilead wrapping up the trial, submitting the proper paperwork and scaling up manufacturing?
“Did remdesivir just ‘solve’ COVID? No,” says Umer Raffat, an analyst at Evercore ISI who has a reason to be bullish on the Gilead news, given that he had an outperform rating on the stock.
Raffat notes that the study specifically excluded patients on mechanical ventilation. Moreover, an earlier study on Chinese patients wasn’t stopped for efficacy reasons. Unlike the Chicago study, the China trial tested patients given a placebo.
“I did a deep dive into the statistics for this interim [China] analysis: remdesivir needed to show [much more than a] 60% effect size vs. placebo to stop at interim…and it clearly didn’t,” he says.
Raffat said there should be cautious optimism around remdesivir, which is administered intravenously.
“Remdesivir is not a silver bullet,” he writes. “Remdesivir is also not a zero (which many investors thought after China studies got paused because of lack of enrollment).”

Jefferies equity analyst Michael J. Yee published in a research note that this Chicago report provides an “incremental positive” but he assumes this significant short-term move in Gilead’s share price will probably pull back a bit given eventual broader awareness that this is not the actual phase three data and that this is overdone in the near term.
J.P.Morgan biotechnology equity analysts wrote in a published research note Thursday that “this clinical trial experience appears to represent another encouraging, albeit largely anecdotal, data point for this high-profile drug candidate.”
“There’s still a great deal we don’t know, and thus we hesitate to put too much into the results generated at a single center without a control group,” said JPMorgan.
“Fortunately, we don’t have to wait long for a Phase 3 readout in severe patients.”
Meanwhile, the Barclays health-care team called the update “encouraging” but note that questions persist.
Barclays adds that data from Gilead’s clinical trials on severe cases due at the end of this month could support approval or expanded use authorization. A treatment is not going to end the pandemic. But it will provide a critical bridge to the ultimate panacea, a vaccine.
Gilead itself says Phase 3 trial data to be available at the end of April and data from other studies to be available in May. “Anecdotal reports, while encouraging, do not provide the statistical power necessary to determine the safety and efficacy profile of remdesivir as a treatment for COVID-19,” the company said.
Separately, India’s Dr. Reddy’s (RDY) is in the early stages of making a generic version of remdesivir, according to a report.
Besides the Gilead news, the other significant story on the coronavirus front was President Donald Trump’s three-stage plan for reopening the economy, which leaves many of the key decisions in the hands of state governors.
Analysts at Bernstein Research pointed out the difficulty the U.S. will have.
“Quality testing technology is now coming together but its implementation on broad scale will likely require 2-3 months. We also do not have the manpower or technologies to adequately follow up on testing results with downstream actions. We don’t have enough public health workers to trace patient contacts through conventional contact tracing, and we also don’t have technologies in place to use digital strategies for tracing contacts and enforcing quarantines. Further, we are just starting the public debate about the material privacy issues that would arise from implementation of these technologies,” they wrote in a note to clients.
The coronavirus pandemic really hasn’t ravaged emerging markets in the same way it has in the U.S. and Europe. JPMorgan strategist Marko Kolanovic points to three major reasons. As the chart shows, the median age in developed markets is much older, and the virus disproportionately affects older people. Emerging markets also tend to have universal BCG vaccinations (to prevent tuberculosis), which seems to be correlated with a reduction of both COVID-19 infection rates and mortalities, he said. Finally, many emerging markets benefit from hot and humid weather that seems to make coronavirus transmission more difficult.

China’s gross domestic product fell 6.8% year-over-year in the first quarter, the country’s statistical office said, in the worst reading since at least 1992. Retail sales in the world’s number-two economy slumped nearly 16% in March, though the pace of industrial production contraction eased to 1.1% in March, from the 13.5% fall in January and February combined.

Analysts polled by Reuters had predicted China’s GDP would shrink by 6.5% in the January to March quarter, compared to a year ago. The forecasts from 57 analysts polled ranged from a 28.9% contraction to a 4% expansion. China’s economy grew 6% in the last quarter of 2019.
Here are some of the key figures released Friday, on a year-over-year basis:
- Industrial production dropped 8.4% in the first quarter, and marked a 1.1% decline in March.
- Fixed-asset investment fell 16.1% in the first quarter.
- Retail sales fell 19% in the first quarter. Sales of consumer goods fell 15.8% in March, while online sales of physical goods rose 5.9%.
More than half of China extended a Lunar New Year holiday by at least a week in an effort to blunt the spread of the virus. Nearly all major industrial enterprises have resumed work, while the return-to-work rate for smaller businesses has topped 80%, according to official reports.
Better data in March could be misleading since it was only then that factories were able to re-open and fulfill some orders from February, said Bo Zhuang, chief China economist at TS Lombard.
“What is really important was that before March, everybody was expecting China to have a V-shaped recovery because it was actually (about) China supply disruption (initially), but now we are seeing this demand shock,” Zhuang told CNBC. “The internal demand shock was massive. That tells us that after coronavirus, even after the lockdowns have been lifted, people are cautious to consume. Shopping malls are open but they are not consuming, and that is the key.”
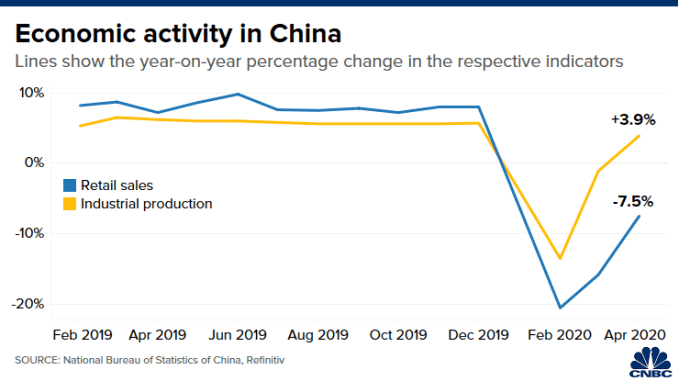
Such a “false dawn” could become apparent soon, Zhuang said, noting that since the virus began to spread around the world in mid-March, demand from rest of the world for Chinese goods has dropped. He expects China’s industrial production and exports to worsen in April or May.
In single-stock headlines Thursday, Goldman Sachs downgraded Apple Inc. (AAPL) to sell on Friday and cut its price target to $233 from $250, as it reduced its earnings estimates for a third time since Feb. 17. Analysts led by Rod Hall said they are modeling a far deeper reduction in unit demand through mid 2020 followed by a shallower recovery heading into 2021. “We also assume some lingering ASP (average selling price) weakness as consumers look to economize similar to what we have seen in prior downturns,” they wrote in a note to clients.

“In addition to this we believe that Services growth slows substantially in 2021 and that Services as a percentage of revenue actually stagnates in that year.”
Goldman is expecting a 36% decline in iPhone unit demand in the second quarter and a 24% decline in the first half of calendar 2020. Analysts are expecting the company’s other products to experience a similar trajectory.
“There are multiple examples of ASPs dropping in the midst of a recession and then remaining weak well beyond the point when units recover,” said the note. Price weakness could affect 5G design choices too, and limited global travel may cause the delay of the launch of this year’s updated iPhone, it said.










